Galvanising
How Galvanising Protects Steel
Galvanisation is the process of coating by which zinc is coated over metal to protect it from corrosion. The idea is quite simple, coating an ordinarily very corrosive metal such as steel with another metal that is less corrosive. To understand why this works requires us to build an understanding of why mteals corrode in the first place.
Corrosion
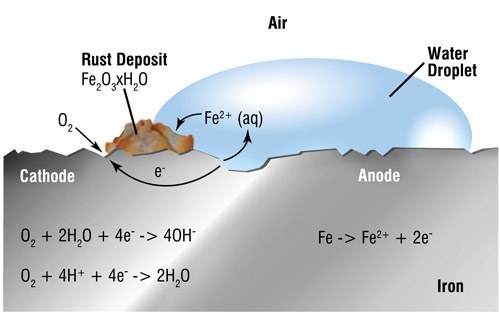
The corrosion of steel is an electrochemical process that requires the simultaneous presence of moisture and oxygen. Essentially, the iron in the steel is oxidised to produce rust. In the absence of either water or oxygen rust cannot occur. The science behind the process is explained below for those who are interested.
Differences in electrical potential are caused on surface areas of exposed steel by non-uniformity of surface composition, by surface moisture or by the electrolyte in which it is immersed. Small electrolytic cells are formed comprising anodes and cathodes. Initial attack occurs at anodic areas on the surface, where ferrous ions go into solution. Electrons are released from the anode and move through the metallic structure to the adjacent cathodic sites on the surface, where they combine with oxygen and water to form hydroxyl ions. These react with the ferrous ions from the anode to produce ferrous hydroxide, which itself is further oxidised in air to produce hydrated ferric oxide (i.e. red rust.) The sum of these reactions can be represented by the following equation:
Fe + 3O2 + 2H2O = 2Fe2O3H2O
(Steel) + (Oxygen) + (Water) = Hydrated ferric oxide (Rust)
Zinc
To avoid corrosion as mentioned we simply want to coat the highly reactive steel with a far less reactive metal such as zinc. Zinc is used as a form of cathodic protection, essentially it is a sacrificial metal however with an extremely slow corrosion rate providing years of protection. Corrosion rate is far slower due to the underlying chemical reaction. When exposed to the atmosphere, the pure zinc (Zn) reacts with oxygen (O2) to form zinc oxide (ZnO), which further reacts with carbon dioxide (CO2) to form zinc carbonate (ZnCO3), a usually dull grey, fairly strong material that protects the steel underneath from further corrosion in many circumstances.
Cathodic protection affords us another advantage over anodising, powder coating, and other finishes, where even when the surface is chipped off or damaged the metal still remains protected due to the zinc acting as a sacrificial anode, drawing the chemical process away from the exposed metal.
Hot Dipping Process
Galvanizing process can be accomplished using many methods(e.g. electroplating) but a more preferred approach is hot-dip galvanisation in which iron parts are directly dipped in molten zinc.
A typical hot-dip galvanizing line operates as follows.
- Steel is cleaned using a caustic solution. This removes oil/grease, dirt, and paint.
- The caustic cleaning solution is rinsed off.
- The steel is pickled in an acidic solution to remove mill scale.
- The pickling solution is rinsed off.
- A flux, often zinc ammonium chloride is applied to the steel to inhibit oxidation of the cleaned surface upon exposure to air. The flux is allowed to dry on the steel and aids in the process of the liquid zinc wetting and adhering to the steel.
- The steel is dipped into the molten zinc bath and held there until the temperature of the steel equilibrates with that of the bath.
- The steel is cooled in a quench tank to reduce its temperature and inhibit undesirable reactions of the newly formed coating with the atmosphere.
Performance
When a hot-dip galvanized object leaves the zinc bath the surface of the object is immediately attacked by oxygen in the air. The resultant oxide layer has very little ability to protect against corrosion. However, water and carbon dioxide in the air quickly change the oxide layer to zinc carbonates. These give a sealed layer with very good adhesion. Since the carbonates have very low solubility in water they give excellent protection to the surface of the zinc coating. The original shiny surface with a metallic lustre disappears to be replaced by matt, light grey colour.
Outdoor air contains greater or lesser amount of corrosive elements — gases, soot, humidity (fog, dew, rain, snow), inert and aggressive dust. Levels can vary with location and the time of the year. Sulphates and sulphites of zinc are water soluble and have poor adhesion to the zinc surface. They are therefore washed away easily by rain. A fresh zinc surface is then exposed to attack by oxygen in the air and the corrosion cycle is repeated. Corrosion in air containing sulphur oxides is therefore greater than in clean air. However, the amount of sulphur dioxide in the atmosphere has decreased drastically during recent years, and consequently zinc corrosion has also decreased.
Warm Dry Atmospheres
Zinc is very stable. The patina formed during initial exposure remains intact preventing further reaction between the galvanized coating and the air, and protection continues indefinitely.
Presence of Atmospheric Moisture
The zinc oxide film is quickly converted to zinc hydroxide, and carbon dioxide normally present in the air reacts to form basic zinc carbonates. These stable inert compounds resist further action and ensure long life for the protective galvanized coating.
Rural Areas
The life of galvanized coatings may be reduced due to the effects of aerial spraying of fertilizers or insecticides. In dry form most fertilizers and insecticides are harmless to zinc coatings but when wetted by rainwater or irrigation spray water, aggressive solutions can be formed which will attack galvanized coatings until washed off by further wetting.
Coastal Areas/Marine
The rate of corrosion is increased by the presence of soluble chlorides in the atmosphere. The performance of galvanized coatings relative to other protective systems is outstanding however, particularly when used as part of a duplex galvanizing-plus-paint system.
Out on the water the corrosion of zinc is influenced by the salt content of the air. However, marine air contains small quantities of magnesium salts, with good passivating influences. Corrosion is therefore not as great as might be expected. The salt content of the air reduces quickly away from the coast.
Industrial Areas
The presence of atmospheric impurities such as sulphurous gases and chemicals results in the formation of soluble zinc salts. These are removed by moisture, exposing more zinc to attack. In light industrial areas galvanized coatings give adequate protection, but in the extremely corrosive conditions of heavy industrial areas it is desirable to reinforce galvanized coatings with a paint system resistant to the prevailing chemical attack.
More Information
Some information courtesy of the following excellent websites: